Latest Updates
-
 Purported Video of Muslim Mob Lynching & Hanging Hindu Youth In Bangladesh Shocks Internet
Purported Video of Muslim Mob Lynching & Hanging Hindu Youth In Bangladesh Shocks Internet -
 A Hotel on Wheels: Bihar Rolls Out Its First Luxury Caravan Buses
A Hotel on Wheels: Bihar Rolls Out Its First Luxury Caravan Buses -
 Bharti Singh-Haarsh Limbachiyaa Welcome Second Child, Gender: Couple Welcome Their Second Baby, Duo Overjoyed - Report | Bharti Singh Gives Birth To Second Baby Boy | Gender Of Bharti Singh Haarsh Limbachiyaa Second Baby
Bharti Singh-Haarsh Limbachiyaa Welcome Second Child, Gender: Couple Welcome Their Second Baby, Duo Overjoyed - Report | Bharti Singh Gives Birth To Second Baby Boy | Gender Of Bharti Singh Haarsh Limbachiyaa Second Baby -
 Bharti Singh Welcomes Second Son: Joyous News for the Comedian and Her Family
Bharti Singh Welcomes Second Son: Joyous News for the Comedian and Her Family -
 Gold & Silver Rates Today in India: 22K, 24K, 18K & MCX Prices Fall After Continuous Rally; Check Latest Gold Rates in Chennai, Mumbai, Bangalore, Hyderabad, Ahmedabad & Other Cities on 19 December
Gold & Silver Rates Today in India: 22K, 24K, 18K & MCX Prices Fall After Continuous Rally; Check Latest Gold Rates in Chennai, Mumbai, Bangalore, Hyderabad, Ahmedabad & Other Cities on 19 December -
 Nick Jonas Dancing to Dhurandhar’s “Shararat” Song Goes Viral
Nick Jonas Dancing to Dhurandhar’s “Shararat” Song Goes Viral -
 From Consciousness To Cosmos: Understanding Reality Through The Vedic Lens
From Consciousness To Cosmos: Understanding Reality Through The Vedic Lens -
 The Sunscreen Confusion: Expert Explains How to Choose What Actually Works in Indian Weather
The Sunscreen Confusion: Expert Explains How to Choose What Actually Works in Indian Weather -
 On Goa Liberation Day 2025, A Look At How Freedom Shaped Goa Into A Celebrity-Favourite Retreat
On Goa Liberation Day 2025, A Look At How Freedom Shaped Goa Into A Celebrity-Favourite Retreat -
 Daily Horoscope, Dec 19, 2025: Libra to Pisces; Astrological Prediction for all Zodiac Signs
Daily Horoscope, Dec 19, 2025: Libra to Pisces; Astrological Prediction for all Zodiac Signs
Covid-19 Vaccines For Kids: DCGI Grants Emergency Use Nod To 3 Vaccines For Different Age Group Of Children
On Tuesday (26 April), the Drugs Controller General of India (DCGI) approved three Covid-19 vaccines for restricted emergency use authorisation (EUA) in children of various ages.
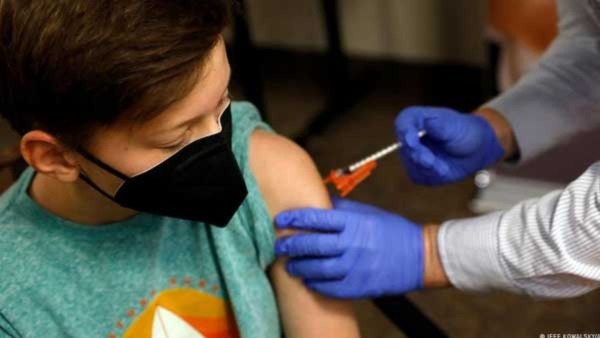
Bharat Biotech's Covaxin received a restricted EUA from the DCGI for children aged 6 to12. Similarly, Biological E's Covid-19 vaccine Corbevax has been approved for children aged 5 to 12 years.
Further, along with Corbevax and Covaxin, the DCGI has also approved Zydus Cadila's Covid-19 vaccine ZyCoV-D for children over the age of 12 years for a two-dose regimen.
This development happened after the Subject Expert Committee's (SEC) discussion of suggestions for the emergency use of Covaxin in children aged 6 to 12 years due to an increase in covid-19 cases in schools.
Covaxin has proven to be effective for adults and is also presently being given to youngsters aged 15 to 18. To give EUA to Covaxin for children aged 6-12 years, experts had requested more information from the corporation, which was delivered on Friday. The SEC was satisfied with the provided data and thus, nods for its EUA among children aged 6-12 years.
As the focus was mainly on the increasing number of cases among children, the SEC has also recommended emergency use of Biological E's Covid-19 vaccine Corbevax and ZyCoV-D.



 Click it and Unblock the Notifications
Click it and Unblock the Notifications












