Latest Updates
-
 A Hotel on Wheels: Bihar Rolls Out Its First Luxury Caravan Buses
A Hotel on Wheels: Bihar Rolls Out Its First Luxury Caravan Buses -
 Bharti Singh-Haarsh Limbachiyaa Welcome Second Child, Gender: Couple Welcome Their Second Baby, Duo Overjoyed - Report | Bharti Singh Gives Birth To Second Baby Boy | Gender Of Bharti Singh Haarsh Limbachiyaa Second Baby
Bharti Singh-Haarsh Limbachiyaa Welcome Second Child, Gender: Couple Welcome Their Second Baby, Duo Overjoyed - Report | Bharti Singh Gives Birth To Second Baby Boy | Gender Of Bharti Singh Haarsh Limbachiyaa Second Baby -
 Bharti Singh Welcomes Second Son: Joyous News for the Comedian and Her Family
Bharti Singh Welcomes Second Son: Joyous News for the Comedian and Her Family -
 Gold & Silver Rates Today in India: 22K, 24K, 18K & MCX Prices Fall After Continuous Rally; Check Latest Gold Rates in Chennai, Mumbai, Bangalore, Hyderabad, Ahmedabad & Other Cities on 19 December
Gold & Silver Rates Today in India: 22K, 24K, 18K & MCX Prices Fall After Continuous Rally; Check Latest Gold Rates in Chennai, Mumbai, Bangalore, Hyderabad, Ahmedabad & Other Cities on 19 December -
 Nick Jonas Dancing to Dhurandhar’s “Shararat” Song Goes Viral
Nick Jonas Dancing to Dhurandhar’s “Shararat” Song Goes Viral -
 From Consciousness To Cosmos: Understanding Reality Through The Vedic Lens
From Consciousness To Cosmos: Understanding Reality Through The Vedic Lens -
 The Sunscreen Confusion: Expert Explains How to Choose What Actually Works in Indian Weather
The Sunscreen Confusion: Expert Explains How to Choose What Actually Works in Indian Weather -
 On Goa Liberation Day 2025, A Look At How Freedom Shaped Goa Into A Celebrity-Favourite Retreat
On Goa Liberation Day 2025, A Look At How Freedom Shaped Goa Into A Celebrity-Favourite Retreat -
 Daily Horoscope, Dec 19, 2025: Libra to Pisces; Astrological Prediction for all Zodiac Signs
Daily Horoscope, Dec 19, 2025: Libra to Pisces; Astrological Prediction for all Zodiac Signs -
 Paush Amavasya 2025: Do These Most Powerful Rituals For Closure On The Final Amavasya Of The Year
Paush Amavasya 2025: Do These Most Powerful Rituals For Closure On The Final Amavasya Of The Year
Cipla, DNDi Launch 4-In-1 Antiretroviral Treatment For Children With HIV
Homegrown pharma major Cipla Ltd and its partner Drugs for Neglected Diseases initiative (DNDi) on Tuesday announced the launch of a 4-in-1 antiretroviral treatment for children living with HIV in South Africa.

South African Health Products Regulatory Authority (SAHPRA) has approved the sweet-tasting, heat-stable and '4-in-1' fixed-dose combination of four antiretroviral (ARV) treatments composed of abacavir, lamivudine, lopinavir, and ritonavir that is specifically designed for infants and young children with HIV, Cipla said in a regulatory filing.
This combination treatment has been developed by Cipla and not-for-profit DNDi, it added. The new formulation does not require refrigeration. It has a sweet taste and is easy to administer to infants and children of different weights and ages, a major improvement for both children and their caregivers over previously available formulations, the company said.
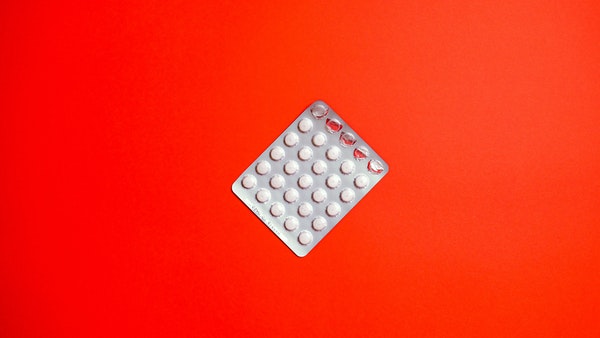
"This approval has come at an important time when so many children are suffering from HIV at such a young age. We will continue in our endeavour to enable access to life-saving solutions for all," Cipla Managing Director and Global CEO Umang Vohra said.
DNDi Head of HIV Irene Mukui said it is highly significant that this first regulatory approval of the 4-in-1 formulation is from a country that has a high burden of HIV among children.
"SAHPRA's accelerated review is notable and encouraging for other high-burden countries, and we acknowledge this show of commitment by the South African authorities. Now, it is our hope that all the necessary steps will be taken - first in South Africa and then in other countries - to ensure the broadest possible access to this optimal formulation for young children who need it," Mukui added.
Until recently, Cipla said the only WHO-recommended lopinavir-based treatment available for babies and very young children in South Africa consisted of a syrup that contained 40 per cent alcohol and required refrigeration.
Caregivers struggled to give this bitter-tasting formulation to young children, leading to poor adherence. Caregivers without refrigeration had a very difficult time storing the formulations - sometimes burying them in the ground to keep them cold, it added.
South Africa has 2.38 lakh children under the age of 15 living with HIV - the highest in the world, the company said.



 Click it and Unblock the Notifications
Click it and Unblock the Notifications












