Latest Updates
-
 Purported Video of Muslim Mob Lynching & Hanging Hindu Youth In Bangladesh Shocks Internet
Purported Video of Muslim Mob Lynching & Hanging Hindu Youth In Bangladesh Shocks Internet -
 A Hotel on Wheels: Bihar Rolls Out Its First Luxury Caravan Buses
A Hotel on Wheels: Bihar Rolls Out Its First Luxury Caravan Buses -
 Bharti Singh-Haarsh Limbachiyaa Welcome Second Child, Gender: Couple Welcome Their Second Baby, Duo Overjoyed - Report | Bharti Singh Gives Birth To Second Baby Boy | Gender Of Bharti Singh Haarsh Limbachiyaa Second Baby
Bharti Singh-Haarsh Limbachiyaa Welcome Second Child, Gender: Couple Welcome Their Second Baby, Duo Overjoyed - Report | Bharti Singh Gives Birth To Second Baby Boy | Gender Of Bharti Singh Haarsh Limbachiyaa Second Baby -
 Bharti Singh Welcomes Second Son: Joyous News for the Comedian and Her Family
Bharti Singh Welcomes Second Son: Joyous News for the Comedian and Her Family -
 Gold & Silver Rates Today in India: 22K, 24K, 18K & MCX Prices Fall After Continuous Rally; Check Latest Gold Rates in Chennai, Mumbai, Bangalore, Hyderabad, Ahmedabad & Other Cities on 19 December
Gold & Silver Rates Today in India: 22K, 24K, 18K & MCX Prices Fall After Continuous Rally; Check Latest Gold Rates in Chennai, Mumbai, Bangalore, Hyderabad, Ahmedabad & Other Cities on 19 December -
 Nick Jonas Dancing to Dhurandhar’s “Shararat” Song Goes Viral
Nick Jonas Dancing to Dhurandhar’s “Shararat” Song Goes Viral -
 From Consciousness To Cosmos: Understanding Reality Through The Vedic Lens
From Consciousness To Cosmos: Understanding Reality Through The Vedic Lens -
 The Sunscreen Confusion: Expert Explains How to Choose What Actually Works in Indian Weather
The Sunscreen Confusion: Expert Explains How to Choose What Actually Works in Indian Weather -
 On Goa Liberation Day 2025, A Look At How Freedom Shaped Goa Into A Celebrity-Favourite Retreat
On Goa Liberation Day 2025, A Look At How Freedom Shaped Goa Into A Celebrity-Favourite Retreat -
 Daily Horoscope, Dec 19, 2025: Libra to Pisces; Astrological Prediction for all Zodiac Signs
Daily Horoscope, Dec 19, 2025: Libra to Pisces; Astrological Prediction for all Zodiac Signs
EU Regulator Approves BioNTech-Pfizer Vaccine For Children In 5-11 Age Group
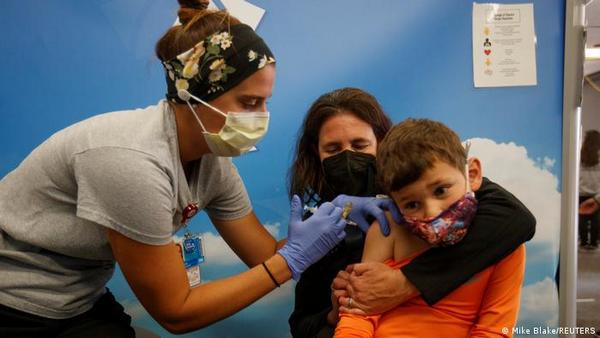
The European Medicines Agency, the EU's regulator for medicinal products, on Thursday approved the BioNTech-Pfizer vaccine, also known as Comirnaty, for children aged 5-11. It's the first vaccine approved for children under 12 in the 27-member EU.The vaccine has already been approved in the EU for use in people between the ages of 12 and 17 years since May and for adults since December 2020.
EMA said the vaccine should be given to children in two doses of 10 micrograms three weeks apart as an injection in the upper arm. Adult doses contain 30 micrograms. "The benefits of Comirnaty in children aged 5 to 11 outweigh the risks, particularly in those with conditions that increase the risk of severe COVID-19," EMA said.
Vaccinating children seen as key
The companies said a clinical trial of children aged 5 to 11 showed their vaccine to be 90.7% effective against the coronavirus. However, the studies have not been large enough to determine whether the vaccine could produce rare side effects such as chest and heart inflammation that have been seen in mostly male older teenagers and young adults after the second dose.
The approval for younger children comes as Europe battles another massive surge of COVID-19 cases. Authorities in Austria, which is seeing a very high rate of infections, did not wait for EMA approval and have already begun vaccinating the 5 to 11 age group.
In Germany and the Netherlands, children now account for the majority of cases, and experts say that vaccinating them could be vital to bringing the fourth wave of the pandemic under control. COVID-19 death in Germany reached 100,000 on Thursday.
What happens now?
EMA approval now has to be rubber-stamped by the EU's executive branch, the European Commission, before the vaccination can be rolled out for the 5-11 age group in EU member states. It typically follows EMA guidelines.
German Health Minister Jens Spahn said earlier this week that shipping of vaccines for younger children in the EU would begin on December 20. Germany's standing vaccination committee (STIKO) is due to issue its recommendation on vaccinating children aged 5-11 before the end of the year.
tj/msh (Reuters, AFP)



 Click it and Unblock the Notifications
Click it and Unblock the Notifications












