Latest Updates
-
 Purported Video of Muslim Mob Lynching & Hanging Hindu Youth In Bangladesh Shocks Internet
Purported Video of Muslim Mob Lynching & Hanging Hindu Youth In Bangladesh Shocks Internet -
 A Hotel on Wheels: Bihar Rolls Out Its First Luxury Caravan Buses
A Hotel on Wheels: Bihar Rolls Out Its First Luxury Caravan Buses -
 Bharti Singh-Haarsh Limbachiyaa Welcome Second Child, Gender: Couple Welcome Their Second Baby, Duo Overjoyed - Report | Bharti Singh Gives Birth To Second Baby Boy | Gender Of Bharti Singh Haarsh Limbachiyaa Second Baby
Bharti Singh-Haarsh Limbachiyaa Welcome Second Child, Gender: Couple Welcome Their Second Baby, Duo Overjoyed - Report | Bharti Singh Gives Birth To Second Baby Boy | Gender Of Bharti Singh Haarsh Limbachiyaa Second Baby -
 Bharti Singh Welcomes Second Son: Joyous News for the Comedian and Her Family
Bharti Singh Welcomes Second Son: Joyous News for the Comedian and Her Family -
 Gold & Silver Rates Today in India: 22K, 24K, 18K & MCX Prices Fall After Continuous Rally; Check Latest Gold Rates in Chennai, Mumbai, Bangalore, Hyderabad, Ahmedabad & Other Cities on 19 December
Gold & Silver Rates Today in India: 22K, 24K, 18K & MCX Prices Fall After Continuous Rally; Check Latest Gold Rates in Chennai, Mumbai, Bangalore, Hyderabad, Ahmedabad & Other Cities on 19 December -
 Nick Jonas Dancing to Dhurandhar’s “Shararat” Song Goes Viral
Nick Jonas Dancing to Dhurandhar’s “Shararat” Song Goes Viral -
 From Consciousness To Cosmos: Understanding Reality Through The Vedic Lens
From Consciousness To Cosmos: Understanding Reality Through The Vedic Lens -
 The Sunscreen Confusion: Expert Explains How to Choose What Actually Works in Indian Weather
The Sunscreen Confusion: Expert Explains How to Choose What Actually Works in Indian Weather -
 On Goa Liberation Day 2025, A Look At How Freedom Shaped Goa Into A Celebrity-Favourite Retreat
On Goa Liberation Day 2025, A Look At How Freedom Shaped Goa Into A Celebrity-Favourite Retreat -
 Daily Horoscope, Dec 19, 2025: Libra to Pisces; Astrological Prediction for all Zodiac Signs
Daily Horoscope, Dec 19, 2025: Libra to Pisces; Astrological Prediction for all Zodiac Signs
Astrazeneca Covid-19 Booster Shot Effective Against Severe Disease, UK Study Finds
The third dose of the AstraZeneca COVID-19 vaccine is effective against symptomatic disease and hospitalisation, offering protection comparable to a booster shot of the Pfizer preventive, according to a study conducted in the UK.

The yet-to-be peer-reviewed study, posted on the preprint repository medRxiv on May 1, assessed the effectiveness of the AstraZeneca (ChAdOx1-S) COVID-19 booster, and compared it with the protection offered by Pfizer (BNT162b2) booster shot.
The researchers from the UK Health Security Agency, London, estimated vaccine effectiveness by analysing data on all adults aged 18 years and above who were vaccinated with the AstraZeneca primary vaccine and either the same or the Pfizer booster vaccine.
A total of 43,171 individuals received an AstraZeneca booster dose, while 13,038,908 individuals received a Pfizer booster dose.
The results show that after 25 or more weeks since primary vaccination with AstraZeneca, vaccine effectiveness against symptomatic disease post-Omicron infection was 8.0 per cent and 19.5 per cent among individuals aged 40 to 64 years and 65 years and above, respectively.
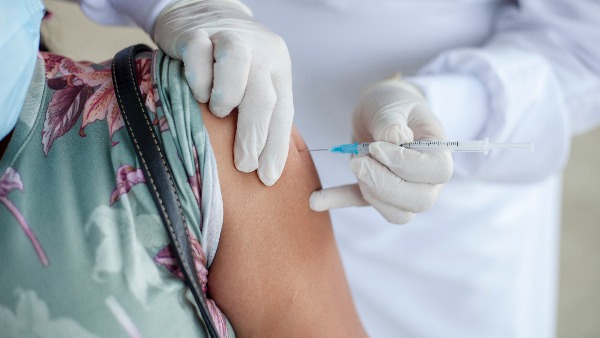
One week after receiving the AstraZeneca booster, the vaccine effectiveness against symptomatic infection was 61.2 per cent among individuals aged 40 to 64 years, while that among Pfizer booster recipients was 58.2 per cent for the same age group.
However, 15 weeks or more after the booster dose, vaccine effectiveness reduced to 37.2 per cent for the AstraZeneca booster and 30.6 per cent for the Pfizer booster. The team observed comparable levels of protection against symptomatic infection among individuals aged 65 years and above.
"Protection against symptomatic disease in those aged 65 years and older peaked at 66.1 per cent and 68.5 per cent amongst those who received the ChAdOx1-S and BNT162b2 booster vaccines, respectively," the authors of the study noted. "Protection waned to 44.5 per cent and 54.1 per cent after 5-9 weeks," they said.
The study found that protection against hospitalisation following Omicron infection peaked at 82.3 after receiving an AstraZeneca booster, as compared to 90.9 per cent for those who received a Pfizer booster.
The research supports the use of the AstraZeneca booster for protection against severe disease with COVID-19 in settings that have not yet offered booster doses, the researchers said.
It suggests that those who received AstraZeneca as a booster in England do not require re-vaccination ahead of others, they said.
Disclaimer: The information provided in this article is for general informational and educational purposes only and is not intended as a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or a qualified healthcare provider with any questions you may have regarding a medical condition.



 Click it and Unblock the Notifications
Click it and Unblock the Notifications












